-
Daphniphyllum alkaloid and iridoid hybrids
Monday, September 30, 2013 -
Hybridaphniphyllines A and B, Daphniphyllum alkaloid and iridoid hybrids suggestive of Diels-Alder cycloaddition in Daphniphyllum longeracemosum
Fei Wang*, Mei-Fen Mao, Guo-Zhu Wei, Yuan Gao, Fu-Cai Ren, Ji-Kai Liu*
Phytochemistry, 2013, 95: 428-435

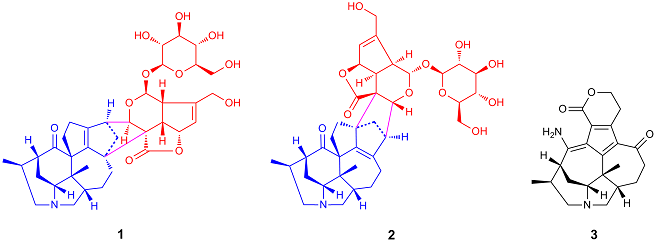
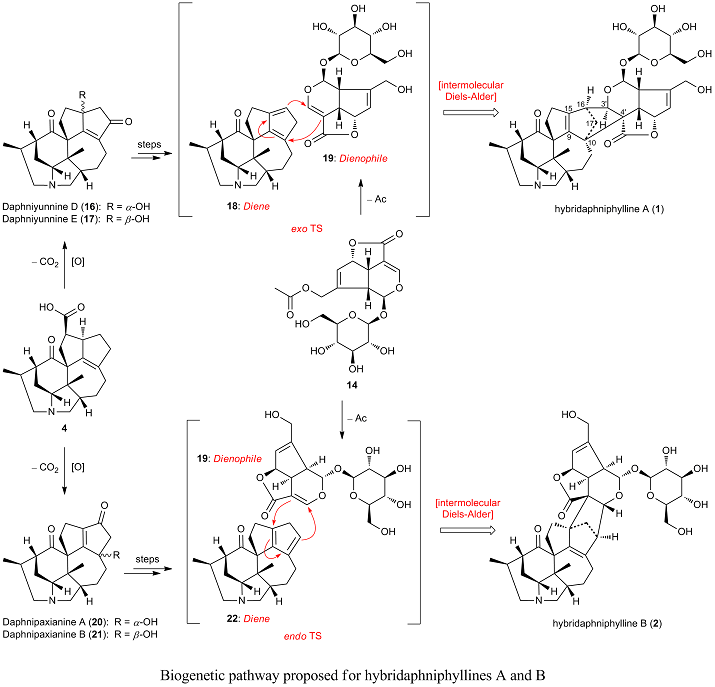
Two Daphniphyllum alkaloid and iridoid hybrids with the hitherto unknown decacyclic fused skeletons, hybridaphniphyllines A (1) and B (2), along with one diamino Daphniphyllum alkaloid, daphnicyclidin I (3), were isolated from dried stems and leaves of Daphniphyllum longeracemosum. Their structures were elucidated on the basis of extensive spectroscopic analysis, and the absolute configuration of daphnicyclidin I was deduced by the CD exciton chirality method. A biogenetic pathway for 1 and 2 involving natural Diels-Alder cycloaddition is proposed.
► Three structurally unusual alkaloids were isolated from Daphniphyllum longeracemosum.
► Their structures were determined by extensive spectroscopic analysis.
► Hybridaphniphyllines A and B represent a hitherto unknown class of natural product hybrids.
► Hybridaphniphyllines A and B were possibly generated via Diels-Alder cycloaddition.

Notes
-
Structure revision of a labdane diterpene zerumin
Wednesday, April 26, 2023 -
Experience sharing —— Unusual carbon chemical shifts
Saturday, March 18, 2023 -
Structure revision of prionitiside A and prionitiside B
Saturday, February 18, 2023 -
JCR 2013 --- Natural Product Chemistry
Thursday, July 31, 2014 -
Daphniphyllum alkaloid and iridoid hybrids
Monday, September 30, 2013 -
JCR 2012 --- Natural Product Chemistry
Thursday, June 20, 2013 -
JCR 2011 --- Natural Product Chemistry
Thursday, July 26, 2012 -
Eudesmanolides from Chloranthus elatior
Tuesday, April 17, 2012 -
Well-known academic journals in natural product chemistry
Thursday, February 16, 2012 -
Structure revision of puerarinoid D
Monday, October 24, 2011